Cimzia Psoriasis Review
January 2020 Report Length. From the Psoriatic Arthritis trials and the phase 2 Psoriasis Trials it is known to be a high performance skin clearing drug.
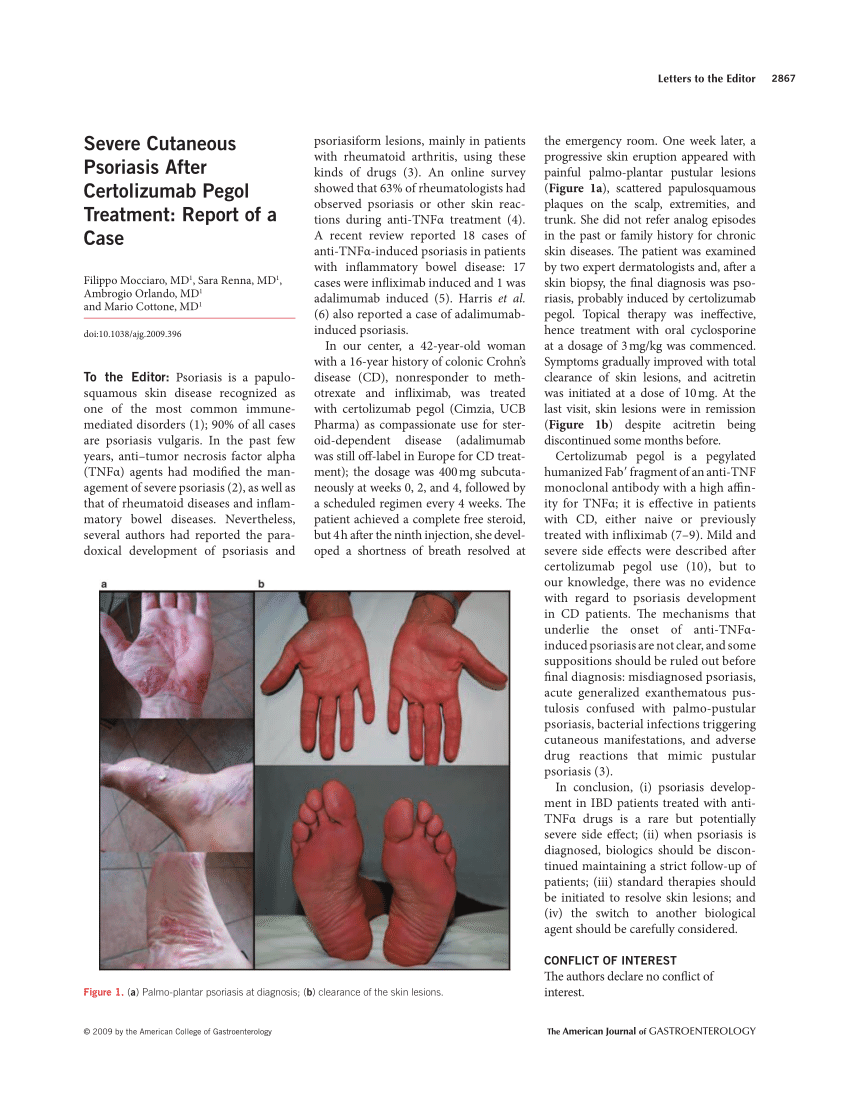
Pdf Severe Cutaneous Psoriasis After Certolizumab Pegol Treatment Report Of A Case
Cimzia is a PEGylated TNF inhibitor.

Cimzia psoriasis review. Cimzia certolizumab reviews from people of your age and gender for uses like Crohns disease Psoriatic arthritis and Rheumatoid arthritis. Final with redactions Publication Date. Ad I Found One Fast Simple Trick.
What are the possible side effects of Cimzia. Certolizumab pegol Cimzia is a PEGylated Fab-only recombinant humanized antibody against TNF-α. By blocking TNF Cimzia can reduce the symptoms of psoriasis.
Show ratings reviews for. PEG is polyethylene glycol a substance that is attached to a protein to make it stay in the body longer. Results from a phase 3 trial demonstrated that certolizumab Cimzia improved psoriasis severity compared with placebo and was noninferior to etanercept Enbrel.
All Conditions 159 reviews Rheumatoid Arthritis 79 reviews Crohns Disease 57 reviews Other 9 reviews Psoriasis. Cimzia Certolizumab received an overall rating of 10 out of 10 stars from 4 reviews. Emuaid Gave Me My Life Back I Am So Thankful For This Amazing Product.
Subcutaneous certolizumab pegol is indicated for the treatment of various immune-mediated inflammatory diseases IMIDs including moderate to severe plaque psoriasis. Is this guidance up to date. In clinical trials 6 out of 10 people taking CIMZIA saw clear or almost clear skin in 16 weeks.
Emuaid Gave Me My Life Back I Am So Thankful For This Amazing Product. Symptoms of psoriasis are more than skin deep. User Reviews for Cimzia.
54 of those users who reviewed Cimzia reported a positive effect while 24 reported a negative effect. In the study participants with moderate to severe plaque psoriasis were randomized to either 400 mg of certolizumab 200 mg of certolizumab or placebo taken every 2 weeks for 16. Certolizumab Pegol Cimzia UCB Canada Inc Indication.
It is not approved for psoriasis but it is current deep into a phase 3 trials for psoriasis. The objective of this report was to perform a systematic review of the beneficial and harmful effects of Certolizumab pegol 400 mg and 200 mg every two weeks administered as an subcutaneous injection for the treatment of adult patients with moderate-to-severe plaque psoriasis. Cimzia is approved for Psoriatic Arthritis and Rheumatoid Arthritis Crohns Disease and Ankylosing Spondylitis.
120 Pages CADTH COMMON DRUG REVIEW. This leads to improvement in symptoms for many people who take it. See what others have said about Cimzia Certolizumab including the effectiveness ease of.
Cimzia targets and blocks tumor necrosis factor TNF a protein involved in the inflammatory response. They can impact many different areas of lifeincluding how you see your future. User Reviews for Cimzia to treat Psoriatic Arthritis Cimzia has an average rating of 47 out of 10 from a total of 13 ratings for the treatment of Psoriatic Arthritis.
Evidence-based recommendations on certolizumab pegol Cimzia for treating moderate to severe plaque psoriasis in adults. 32 of people who took 400 mg of Cimzia every 2 weeks. Treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.
Patients should be closely monitored for the signs and symptoms of infection during and after treatment with CIMZIA. Emuaid Defeats Severe Psoriasis. A Review in Moderate to Severe Plaque Psoriasis.
Clinical Review Report. Ad I Found One Fast Simple Trick. There is a complex patient access scheme for certolizumab pegol which provides the first 12 weeks of certolizumab pegol free of charge.
Cimzia helps lower the amount of TNF alpha to more normal levels and switches off the inflammatory cycle of psoriasis and or psoriatic arthritis. Important Safety Information You Should Know About CIMZIA certolizumab pegol Serious and sometimes fatal side effects have been reported with CIMZIA including tuberculosis TB bacterial sepsis invasive fungal infections such as histoplasmosis and infections due to other opportunistic pathogens such as Legionella or Listeria. 31 of those users who reviewed Cimzia reported a positive effect while 54 reported a negative effect.
Emuaid Defeats Severe Psoriasis. Those with results at 16 weeks maintained skin clearance for nearly 1 year. CADTH Common Drug Review Version.
In clinical studies of Cimzia for psoriasis injection site reactions were reported in. 17 of people who took 200 mg of Cimzia every 2 weeks.

Certolizumab Pegol In Plaque Psoriasis Considerations For Pregnancy

Cimzia S Value In Patients With Psoriasis Demonstrated

See The Trials Pso Cimzia Certolizumab Pegol

Cimzia Effectiveness Assessed For Chronic Plaque Psoriasis Mpr